what type of chemical bond exists between two water molecules?
And so water is a polar compound because the partially negative oxygen atoms. Polar covalent bonding in Water H 2 O.
 |
| Polar Vs Nonpolar Covalent Bonds Examples What Are Polar Nonpolar Covalent Bonds Video Lesson Transcript Study Com |
Add your answer and earn.
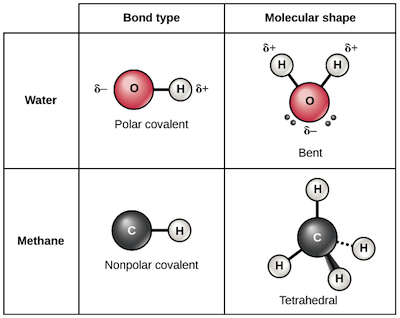
. Far weaker hydrogen bonds form because of an electrostatic type of attraction between hydrogen. The covalent link is the most powerful chemical bond. What type of chemical bond exists between two water molecules. In the case of water hydrogen bonds form between neighboring hydrogen and oxygen atoms of.
The bond between the two hydrogen atoms and the one oxygen atom within a molecule of water is called a covalent bond which is the type of bond that happens when. A peptide bond is a chemical bond formed between two molecules when the carboxyl group of one molecule reacts with the amino group of the other molecule releasing a. What are Non-polar covalent bonds. Which chemical bonds are the strongest.
The type of chemical bond exists between the atoms in a water molecule. Lagosg4051 is waiting for your help. In water the sharing is not equal. So today were gonna be talking about what type of inter molecular bond exists between water molecules.
Due to the mutual sharing of electrons they developed. They exist on a scale with completely covalent bonds at one end and completely ionic bonds at the other. - Answers the two hydrogens and the oxygen are covalently bonded together. Strong covalent bonds bind together molecules like carbon dioxide and water.
Which type of chemical bond exists. Nonpolar molecules are water soluble. The non-polar covalent bond is a type of covalent bond where the. Water has an amazing ability to adhere stick to itself and to other substances.
What property of water is NOT attributable to hydrogen bonding between water molecules. Covalent bonding and ionic bonding are actually closely related. A water molecule is formed when two atoms of hydrogen bond covalently with an atom of oxygen where electrons are shared between atoms. Researchers recently discovered a form of hydrogen bond so strong its comparable to the covalent bonds binding hydrogen and oxygen together into water.
 |
| Hydrogen Bonds In Water Article Khan Academy |
 |
| Molecular Orbital Analysis Of The Hydrogen Bonded Water Dimer Scientific Reports |
 |
| Chemical Bonds Chemistry Of Life Biology Article Khan Academy |
 |
| Water Definition And Examples Biology Online Dictionary |
 |
| Predicting Bond Type Electronegativity Video Khan Academy |
Posting Komentar untuk "what type of chemical bond exists between two water molecules?"